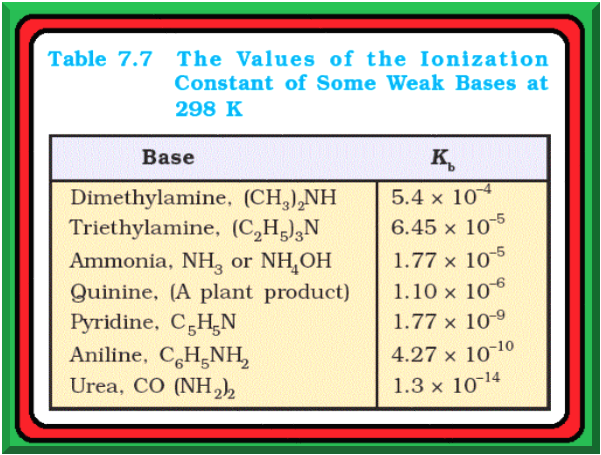
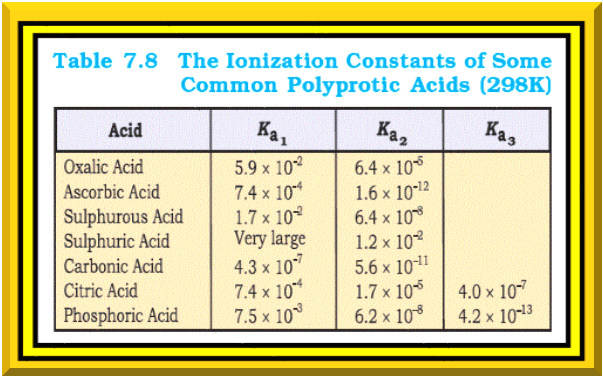
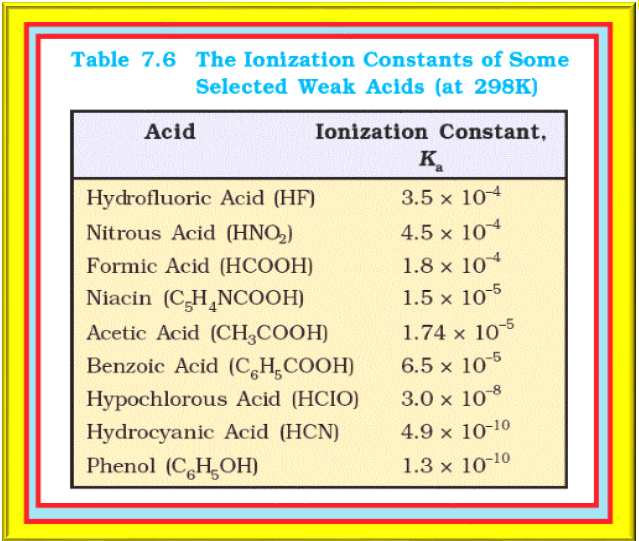
Ionization Constants of Weak Acids
`=>` Consider a weak acid `color{red}(HX)` that is partially ionized in the aqueous solution. The equilibrium can be expressed by:
`color{red}(HX (aq) + H_2O (l) ⇌ H_3 O^+ (aq) + X^- (aq))`
`color{green}("Initial concentration (M)")`
`color{red}(c \ \ \ \ \ \ \ \ 0 \ \ \ \ \ \ \ \ \ \ \ 0)`
`color{green}("Let α be the extent of ionization
Change (M)")`
`color{red}(-c alpha \ \ \ \ \ \ \ \ \ \ +c alpha \ \ \ \ \ \ \ \ \ \ +c alpha)`
`color{green}("Equilibrium concentration (M)")`
`color{red}(c- calpha \ \ \ \ \ \ \ \ \ \ c alpha \ \ \ \ \ \ \ \ \ \ c alpha)`
Here, `color{red}(c)` = initial concentration of the undissociated acid, `color{red}(HX)` at time, `color{red}(t = 0. α =)` extent up to which `color{red}(HX)` is ionized into ions. Using these notations, we can derive the equilibrium constant for the above discussed acid dissociation equilibrium:
`color{red}(K_a = c^2 alpha^2 // c (1-alpha) = c alpha^2 // 1- alpha)`
`color{red}(K_a)` is called the dissociation or ionization constant of acid `color{red}(HX)`. It can be represented alternatively in terms of molar concentration as follows,
`color{red}(K_a = [H^+] [X^- ] // [HX])` ................(7.30)
`=>` At a given temperature `color{red}(T, K_a)` is a measure of the strength of the acid `color{red}(HX)` i.e., larger the value of `color{red}(K_a)`, the stronger is the acid. `color{red}(K_a)` is a dimensionless quantity with the understanding that the standard state concentration of all species is `1M`.
The values of the ionization constants of some selected weak acids are given in Table 7.6. The `color{red}(pH)` scale for the hydrogen ion concentration has been so useful that besides `color{red}(pK_w)`, it has been extended to other species and quantities. Thus, we have:
`color{red}(pK_a = -log(K_a))` .........(7.31)
Knowing the ionization constant, `color{red}(K_a)` of an acid and its initial concentration, `color{red}(c)`, it is possible to calculate the equilibrium concentration of all species and also the degree of ionization of the acid and the `color{red}(pH)` of the solution.
`=>` A general step-wise approach can be adopted to evaluate the `color{red}(pH)` of the weak electrolyte as follows:
Step 1. The species present before dissociation are identified as Brönsted-Lowry acids / bases.
Step 2. Balanced equations for all possible reactions i.e., with a species acting both as acid as well as base are written.
Step 3. The reaction with the higher `color{red}(K_a)` is identified as the primary reaction whilst the other is a subsidiary reaction.
Step 4. Enlist in a tabular form the following values for each of the species in the primary reaction
`color{red}(a)` Initial concentration, `color{red}(c)`.
`color{red}(b)` Change in concentration on proceeding to equilibrium in terms of `color{red}(α)`, degree of ionization.
`color{red}(c)` Equilibrium concentration.
Step 5. Substitute equilibrium concentrations into equilibrium constant equation for principal reaction and solve for `color{red}(α)`.
Step 6. Calculate the concentration of species in principal reaction.
Step 7. Calculate `color{red}(pH = – log[H_3O^+])`
`color{red}(HX (aq) + H_2O (l) ⇌ H_3 O^+ (aq) + X^- (aq))`
`color{green}("Initial concentration (M)")`
`color{red}(c \ \ \ \ \ \ \ \ 0 \ \ \ \ \ \ \ \ \ \ \ 0)`
`color{green}("Let α be the extent of ionization
Change (M)")`
`color{red}(-c alpha \ \ \ \ \ \ \ \ \ \ +c alpha \ \ \ \ \ \ \ \ \ \ +c alpha)`
`color{green}("Equilibrium concentration (M)")`
`color{red}(c- calpha \ \ \ \ \ \ \ \ \ \ c alpha \ \ \ \ \ \ \ \ \ \ c alpha)`
Here, `color{red}(c)` = initial concentration of the undissociated acid, `color{red}(HX)` at time, `color{red}(t = 0. α =)` extent up to which `color{red}(HX)` is ionized into ions. Using these notations, we can derive the equilibrium constant for the above discussed acid dissociation equilibrium:
`color{red}(K_a = c^2 alpha^2 // c (1-alpha) = c alpha^2 // 1- alpha)`
`color{red}(K_a)` is called the dissociation or ionization constant of acid `color{red}(HX)`. It can be represented alternatively in terms of molar concentration as follows,
`color{red}(K_a = [H^+] [X^- ] // [HX])` ................(7.30)
`=>` At a given temperature `color{red}(T, K_a)` is a measure of the strength of the acid `color{red}(HX)` i.e., larger the value of `color{red}(K_a)`, the stronger is the acid. `color{red}(K_a)` is a dimensionless quantity with the understanding that the standard state concentration of all species is `1M`.
The values of the ionization constants of some selected weak acids are given in Table 7.6. The `color{red}(pH)` scale for the hydrogen ion concentration has been so useful that besides `color{red}(pK_w)`, it has been extended to other species and quantities. Thus, we have:
`color{red}(pK_a = -log(K_a))` .........(7.31)
Knowing the ionization constant, `color{red}(K_a)` of an acid and its initial concentration, `color{red}(c)`, it is possible to calculate the equilibrium concentration of all species and also the degree of ionization of the acid and the `color{red}(pH)` of the solution.
`=>` A general step-wise approach can be adopted to evaluate the `color{red}(pH)` of the weak electrolyte as follows:
Step 1. The species present before dissociation are identified as Brönsted-Lowry acids / bases.
Step 2. Balanced equations for all possible reactions i.e., with a species acting both as acid as well as base are written.
Step 3. The reaction with the higher `color{red}(K_a)` is identified as the primary reaction whilst the other is a subsidiary reaction.
Step 4. Enlist in a tabular form the following values for each of the species in the primary reaction
`color{red}(a)` Initial concentration, `color{red}(c)`.
`color{red}(b)` Change in concentration on proceeding to equilibrium in terms of `color{red}(α)`, degree of ionization.
`color{red}(c)` Equilibrium concentration.
Step 5. Substitute equilibrium concentrations into equilibrium constant equation for principal reaction and solve for `color{red}(α)`.
Step 6. Calculate the concentration of species in principal reaction.
Step 7. Calculate `color{red}(pH = – log[H_3O^+])`